The respected Comrade
"We should make it our major thrust to develop such core, basic technologies as IT, nanotechnology and bioengineering, along with such pivotal and beneficial scientific and technological fields as new materials and energy, space and nuclear technologies, and concentrate our efforts on them."
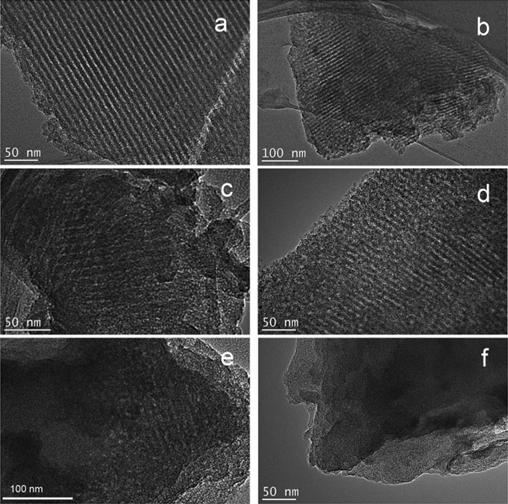
We investigated the effects of post-KOH activation on pore textural characteristics of nitrogen-containing mesoporous carbon. Adjustment KOH/carbon weight ratio and activation temperature can lead to remarkable increases in BET surface area and total volume up to 1652 m2/g and 0.92 cm3/g, under the existence of periodicity of mesopore.
In recent years, porous carbon materials with ordered mesostructure have gained much scientific attention in clean energy tenchnologies such as adsorption/separation, catalyst supports, supercapacitor electrods and sensor electrodes because of their uniform alignment of ordered channels, mechanical and chemical stabilities.
The most widespread activation approach of initial mesoporous carbons is based on physical activation in the presence of steam, oxygen or carbon dioxide at high temperature and chemical activation involving the impregnation of potassium hydroxide (KOH), sodium hydroxide (NaOH), zinc chloride (ZnCl2), phosphoric acid (H3PO3) etc. in an inert aeration usually under nitrogen atmosphere, similar to the methods for the commercial production of activated carbons.
In present study, the KOH activation of nitrogen-containing porous carbon and a carbonization process were systematically investigated to determine the optimized activation conditions for CO2 capture.
The KOH activation was carried out in the temperature range of 700–850 °C with the activation time of 1 h, while KOH/carbon weight ratio was adjusted as 4.0 and 6.0, respectively.
The resulting data showed the KOH activation process afforded porous carbons with high specific surface areas and large total pore volumes, meanwhile, mesoshapes with order channels can be still preserved under conditions with activation temperature up to 800 °C and KOH/carbon weight ratio of 4.0.
It is found that activated nitrogen-containing mesoporous carbons activated under the optimized condition shows excellent CO2 capture (3.12 mmol/g, 298 K, 0.95 bar) and selectivity (18.5, 298 K). This material may be a promising candidate for absorbent for CO2 capture.
In 2020, the above results were published on "Chemical Physics Letters" under the title of "Post-KOH activation of nitrogen-containing porous carbon with ordering mesostructure synthesized through a self-assembly" (https://doi.org/10.1016/j.cplett.2019.137028).