The respected Comrade
"Great efforts should be made in the field of cutting-edge science and technology to develop technologies with a world-class competitive edge."
111In that has relatively short half-life of 2.805d is converted into stable 111Cd with electron capture (99%). While gamma-rays with two diferent energies of 0.171MeV and 0.245MeV is emitted from 111In. This radioactive isotope is an appropriate one to nuclear pharmaceuticals.
111In could be synthesized by irradiating protons onto a natural cadmium or natural tin target in an accelerator, or by irradiating α-participle of 30MeV on natural silver target in the U-200 accelerator (FLNR, JINR).
In the production of 111In for nuclear pharmaceutical purpose, removing of radioactive indium from cadmium solution and ensuring the radiochemical purity of 111In compound were very important.
Separation of 111In for nuclear pharmaceutical purpose from irradiated Cd target could be carried out by using various physicochemical methods such as ion exchange and solvent extraction, as well as solid phase extraction chromatography coupled with these methods.
The separation of In (III), Ga (III) and Zn (II) from sulfuric acid solutions using the extractant polymer resin contained 2-ethylhexyl phosphoric acid mono(2-ethylhexyl) ester (P507 extraction resin) was investigated.
However, there were few reported data about the extraction equilibrium constants, adsorption band movement rates of the metal ions and the separation of metal ions under the given experimental conditions using D2EHPA-St-DVB extractant resin.
In this paper, authors reported investigating results of evaluating the extraction equilibrium constants and adsorption band movement rates of the elements under the given experimental conditions using D2EHPA-St-DVB extractant resin, and the separation method of 111In from Cd target irradiated from charged particle accelerator.
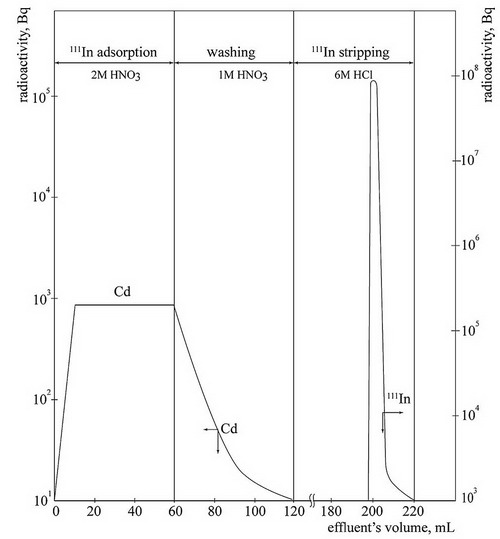
A piece of irradiated Cd target was dissolved in 15 mL of concentrated nitric acid. After the target solution corrected for the acid concentration to 2 mol•L−1HNO3, was filtered with quantitative filter paper and was adsorbed onto the column, the column was washed with 1 mol•L−1HNO3. (Washing was done until no Cd was detected by radioactivity measuring.) After the washing process, 111In was stripped with 6 mol•L−1HCl solution. Iron contained in the eluent (FeCl4−) was removed by using anion exchange resin (Dowex 1 × 4), and the solution was evaporated to dryness. And then 111In was leached by adding 0.05mol•L−1 HCl.
The identification of nuclear purity was carried out with 4096 channel analyzer (Aptec) combined with Ge (Li) semiconductor detector.
With established extraction chromatography, 111InCl3 can be produced to meet nuclear medical requirements.
The results of this study were published in Springer's Journal of Radioanalytical and Nuclear Chemistry (2022), under the title "Radiochemical separation of 111In from irradiated cadmium targets by D2EHPA‑St‑DVB extractant impregnated resin"(https://doi.org/10.1007/s10967-022-08549-x).