The respected Comrade
"We should make it our major thrust to develop such core, basic technologies as IT, nanotechnology and bioengineering, along with such pivotal and beneficial scientific and technological fields as new materials and energy, space and nuclear technologies, and concentrate our efforts on them."
In our research, it was illustrated that graphitic carbon nitride (GCN) assisted by oxalic acid (OA) improve photo-catalytic activity largely by using first-principles study.
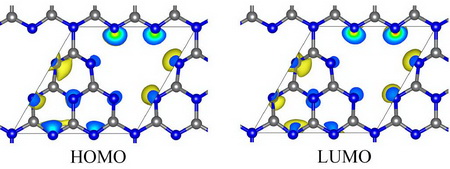
Figure 1 shows HOMO and LUMO of GCN, in which both HOMO and LUMO are concentrated around nitrogen atom of GCN, which means that photo-induced electronic transition occurs between nitrogen atoms, and therefore, photo-induced electron and hole are easy to recombine.
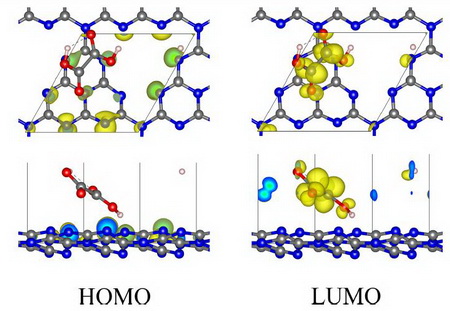
Figure 2 shows HOMO and LUMO of OAGCN (GCN assisted by OA), in which HOMO is concentrated around nitrogen atoms of GCN, and LUMO is concentrated around OA molecule, which means that photo-induced electronic transition occurs from nitrogen atom of GCN to OA molecule, and therefore, photo-induced electron and hole are more difficult to recombine than GCN.
To investigate photo-induced electron-hole recombination more quantitatively, effective masses, static dielectric constants and excitonic binding energies of GCN and OAGCN structures were calculated. OAGCN structure has smaller effective masses of electron and hole than GCN, and it is known that a smaller effective mass of photo-induced charge carriers induces higher probability of reaching the surface reaction sites, hence enhancing photo-catalytic activity, which suggests that OAGCN has more enhanced photo-catalytic activity than GCN. Also, static dielectric constant of OAGCN is larger than GCN, because OA molecule induces high polarity. Larger static dielectric constant and smaller reduced effective mass result in smaller excitonic binding energy, and therefore, OAGCN has smaller excitonic binding energies than GCN, which means that OAGCN has lower recombination rate of photo-induced charge carriers than GCN.
Ionization potential and electron affinity were calculated. Ionization potential and electron affinity of GCN calculated by using Carter's method were in good agreement with experimental value, and therefore, it can be said that those of OAGCN calculated by using Carter's method are believable. Calculation result shows that ionization potential and electron affinity of OAGCN are about 0.5 eV lower than those of GCN.
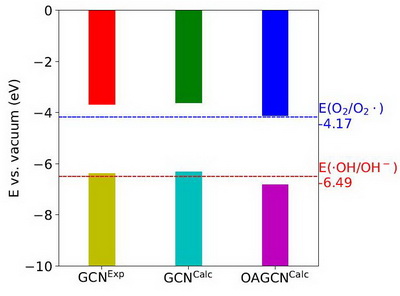
Figure 3 shows VBM and CBM levels of GCN and OAGCN with the redox potentials of O2/O2∙and OH/OH-. The CBM level of GCN is higher than the redox potential of O2/O2∙, and therefore, the electron at CBM can reduce O2 to O2∙, while its VBM level is higher than the redox potential of ∙OH/OH-, and therefore, the hole at VBM can't oxidize OH-to OH. For OAGCN, its CBM level is a little higher than the redox potential of O2/O2∙, and its VBM level is lower than the redox potential of∙OH/OH-, which means that the electron at CBM can reduce O2 to O2, and the hole at VBM can oxidize OH to OH-. Totally, for GCN, only the electron at CBM contributes to produce radicals for removing pollutants, while for OAGCN, both the electron at CBM and the hole at VBM contribute to produce radicals for removing pollutants, which is one of the reasons why the photo-catalytic activity of OAGCN is higher than GCN for removing pollutants.
Our results of this study were published in the journal "Journal of Molecular Modeling" under the title of "Ab initio study of photocatalytic characteristics of graphitic carbon nitride assisted by oxalic acid" (https://doi.org/10.1007/s00894-021-04858-2).