The great leader Comrade
"Mathematics, physics, biology and other basic sciences should be developed so that they can render active services to developing the country's science and technology."
Amyloid aggregation is a process by which proteins are assembled into fibrils characterized by a high content of β-sheets. The formation of fibrils is associated with neurodegenerative diseases such Alzheimers and Parkinsons. In the past decades, there has been a lot of experimental evidences showing that small oligomers and protofibrils in the early stages of fibril aggregation, are in fact, more toxic. An understanding of the physical and chemical processes associated with the initial stages of amyloid aggregation may help with working out medical therapies that can intervene at earlier stages of the disease development.
The stability of amyloid fibrils has typically been rationalized by the presence of a dense network of hydrogen bonds formingβ-sheet structures. However, the recent experiments using solid-state NMR spectroscopy, show that 3D structure of Aβ1-42 fibrils is also characterized by a hydrophobic core made up of maximally buried hydrophobic side chains. Moreover, it has also been shown that the toxicity of the oligomers increases with the increase of hydrophobicity.
The interplay between the hydrophobic interactions among the side chains and the hydrophilic interactions through the dense hydrogen bond network and its role in amyloid aggregation still remains an open question. One of the obvious experimental challenges in characterizing the mechanisms associated with aggregation like those discussed earlier, is the ability to monitor the aggregation process. In this regard, there has been an increasing effort to understand the optical properties of amyloid proteins. Recent experimental studies have shown that amyloid fibrils develop an intrinsic fluorescence during aggregation. It has also been observed that these protein aggregates can absorb low energy photons in the energy range of 250-500nm with the measured excitation peak at around 365nm. Remarkably, these features do not require the presence of aromatic amino acids. These anomalous optical properties may not be exclusive to amyloid aggregates. The fluorescent experimental studies for a series of non-aromatic biogenic and synthetic peptides based on alanine, valine and isoleucine also have suggested that the intrinsic fluorescence in the aggregated state/condensed phase is associated with the abundant existence of hydrogen bonding between amide groups. Recently, Prasad et al. showed that a monomeric protein devoid of aromatic residues features significant absorption between 250-300nm and a long tail in the absorption up to about 800nm. Using electronic structure calculations, they proved that the charged amino (NH3+) and carboxylate (COO-) of the spatially proximal lysine and glutamic acid side chains, act as electronic charge acceptors or donors for photo induced electron transfer either from or to the polypeptide backbone or to each other. They also demonstrated that charge-transfer (CT) excitation involving these charged groups and the peptide backbone appear to be the main factor which causes the optical activity of these proteins in the range between 220-380nm. In specific cases, the increase of the intrinsic fluorescence during the aggregation for the model system of insulin and lysozyme suggest this phenomenon is at least partially caused by the chemical process like oxidation. It is shown that this intrinsic fluorescence might be utilized as a label-free diagnostic tool to probe the structural and dynamical transition of amyloid-like aggregates. For example, Ansari et al. showed that the absorption intensities of the PEST fragment of human c-Myc and its mutant at wavelengths of 250-800nm are dependent on the 3D proximity of the charged functional groups across the protein. Moreover, the significant changes in the spectra by changing pH in the range of 3-11 as well as by the application of different temperatures and salts show a strong correlation between their secondary structure and fluorescence. The increase of the absorbance spectra with time in Hen Egg-White Lysozyme at pH of 2 is directly correlated with the growth of aggregates, as confirmed by the increasing thioflavin T fluorescence. Meanwhile, the sensitivity of absorption spectra by the charge transfer transition to the proximity of the different charged groups is also very supportive to the feasibility to employ the non-aromatic residues as new luminescent biomolecules.
In our work, we combine fluorescence spectroscopy experiments and theoretical modeling to specifically examine the role of termini interactions on the optical properties. Optical absorption and fluorescence is measured for a six-chain amino acid 2Y3J (AIIGLM) which forms a segment of the full amyloid beta 1-40. In order to explore the sensitivity of the optical properties to the termini interactions, the experiments were repeated by acetylating the N-terminus. Although atomic force microscopy experiments indicate the formation of some form of fibrilar or crystal aggregates in both systems, the optical properties are strikingly different – acetylation significantly reduces optical activity between 280-350nm.
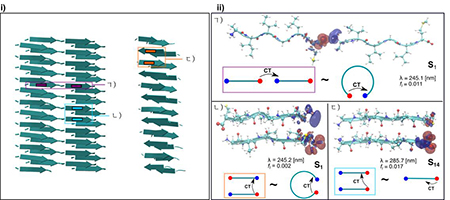
Linear (a), Anti-parallel (b) and Parallel (c). The red and blue colors represent positive (electron) and negative (hole) values of the iso-surface which are 0.002a.u.
In the earlier studies, we have shown using density functional theory (DFT) based calculations, that strong hydrogen bonding interactions between the N and C termini of the amyloid proteins, play an important role in turning their optical properties. Due to the complexity of the amyloid fibril morphology, these calculations were done using standard generalized gradient approximation (GGA) functional on small model crystal structures. When used to determine optical properties, GGA functional can often suffer from inadequately capturing the physics of CT excitations. Here, we use range corrected hybrid functional to examine the electronic character of the optical excitations. We suggest that the low energy excitations observed in the experiment appear to be modulated by the extent of hydrogen bonding interactions which is affected by the specific conformations of the peptide. The low energy absorption of photons involves a mixture of CT excitation from C-to-N terminus and C-terminus to backbone groups. This region of the optical spectrum may thus provide a way to examine the extent of packing and specific hydrogen bond interactions in peptide aggregates.
Our research achievements have already been reviewed in the journal of "Phys. Chem. Chem. Phys." under the title of "Amyloid Aggregates and their Optical Properties"(https://doi.org/10.1039/c9cp04648h).